Three commonly confused words. In order to understand information correctly, it is necessary to grasp the meaning of each word.
To illustrate using a bonfire analogy
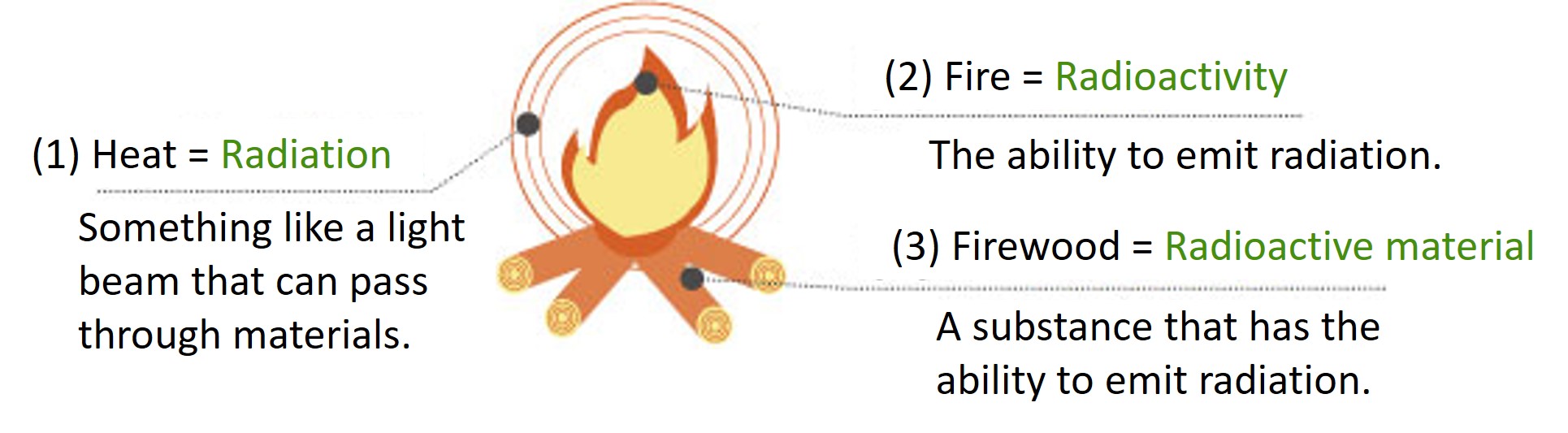
Types of radiation and penetrating power
The ability of radiation to penetrate through materials varies depending on its type. To block radiation effectively, it is necessary to use materials corresponding to its penetrating power.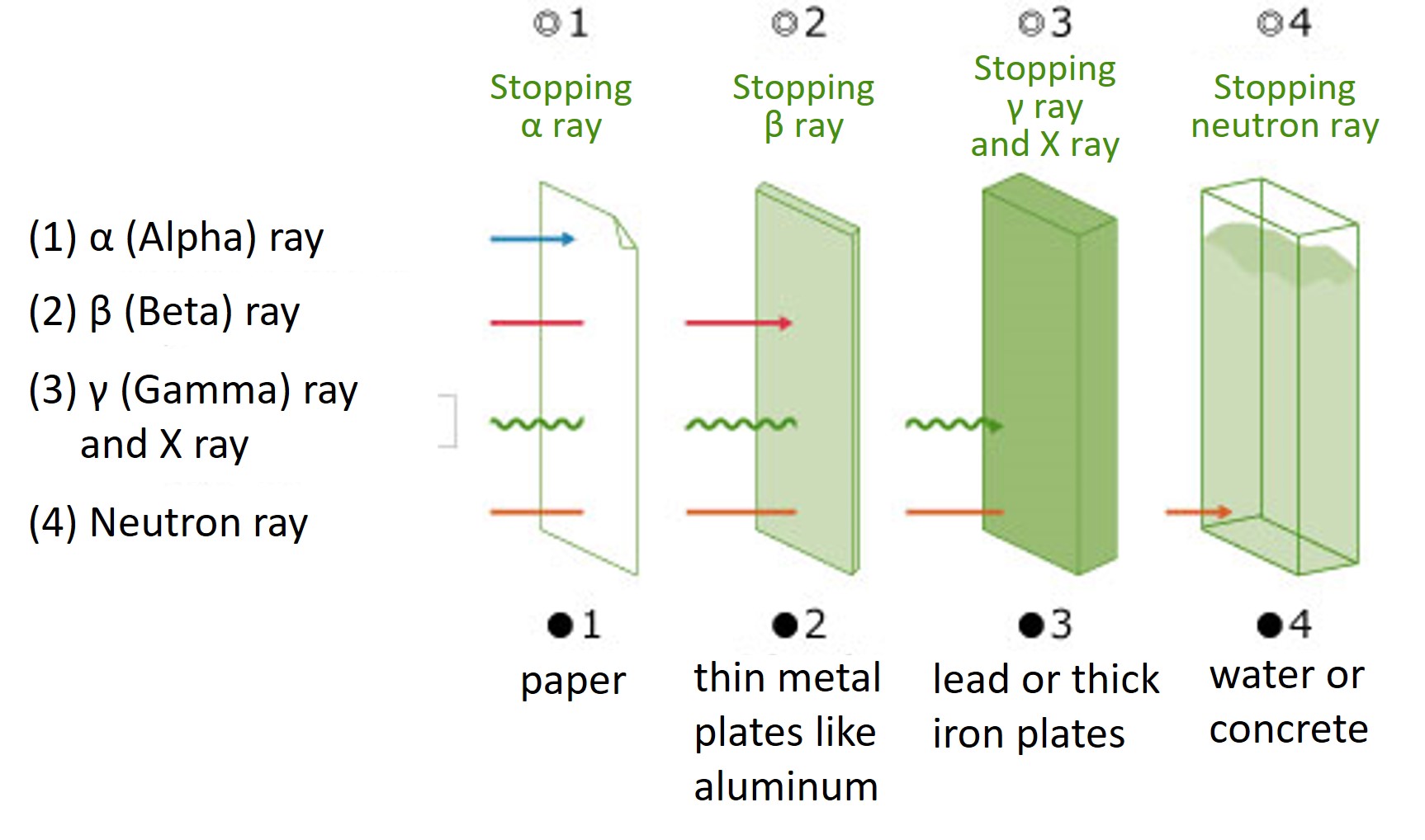
Units of radiation
Since the type and amount of energy of the radiation emitted varies according to the type of radioactive material, it is necessary to compare the degree of effect of radiation on the human body using the unit "Sievert" (Sv), which represents the level of effect on the human body, rather than the unit "Becquerel" (Bq).
Regarding half-life
All substances are composed of atomic nuclei and the electrons that surround them. Radioactive materials, such as uranium, have an unstable balance between their atomic nuclei and electrons, causing them to emit radiation in order to reach a stable state. This transformation is known as "decay", and the level of radioactivity is halved after a certain period of time. The term "half-life" refers to the time it takes for the number of initial atomic nuclei to be reduced by half through decay. The half-life varies depending on the type of radioactive material, ranging from over 10 billion years to mere seconds. (*The image is for illustrative purposes only.)
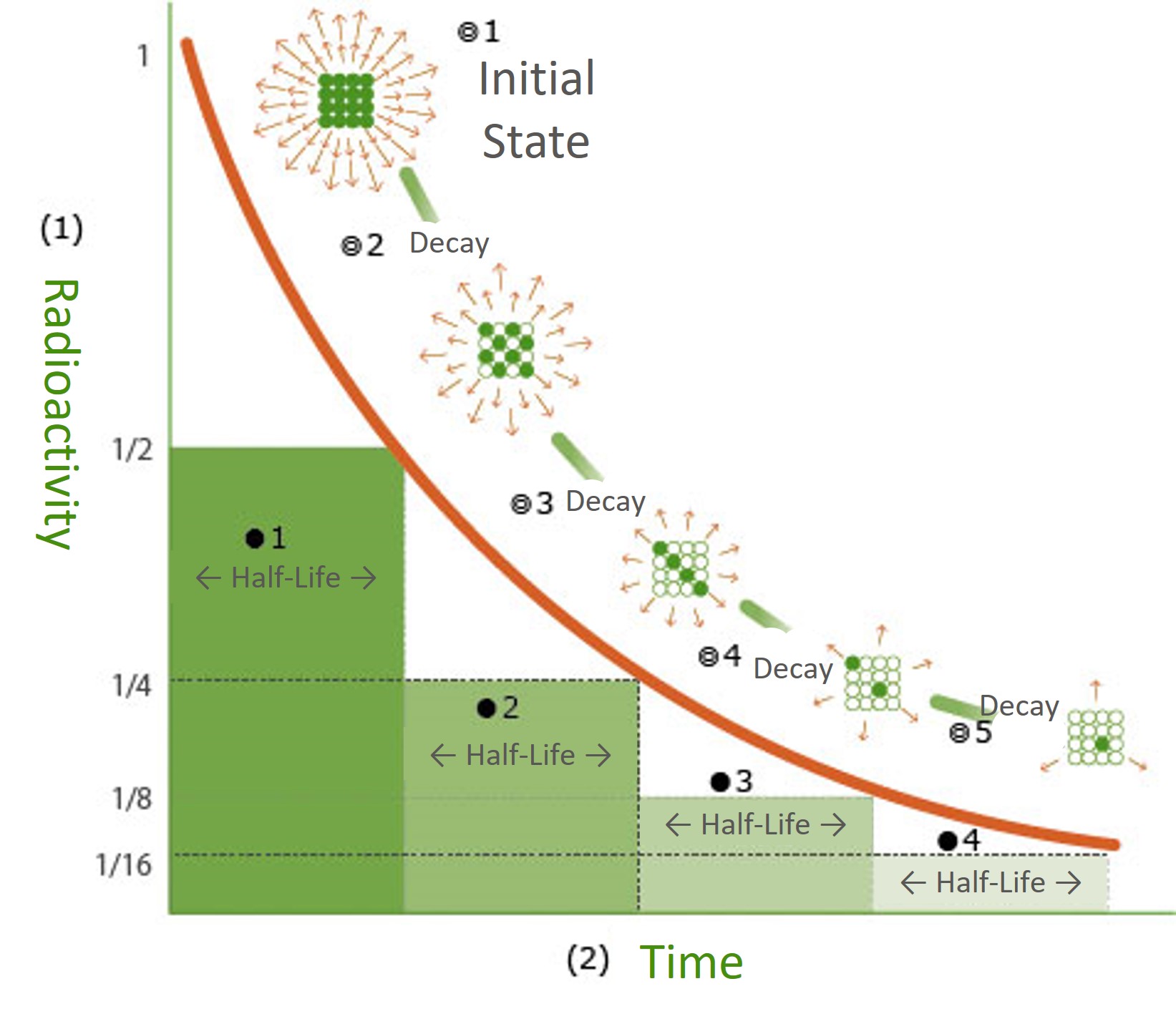
(*The image is for illustrative purposes only.)
| Radioactive materials(Radioactive Elements) | Radiation Emitted* | Physical Half-Life |
|---|---|---|
| Thorium-232 | Alpha, Beta, Gamma | 14.1 billion years |
| Uranium-238 | Alpha, Beta, Gamma | 4.5 billion years |
| Potassium-40 | Beta, Gamma | 1.3 billion years |
| Carbon-14 | Beta | 5,700 years |
| Cesium-137 | Beta, Gamma | 30 years |
| Strontium-90 | Beta | 29 years |
| Cesium-134 | Beta, Gamma | 2 years |
| Iodine-131 | Beta, Gamma | 8 days |
| Radon-220 | Alpha, Gamma | 56 seconds |
*Includes radiation from decay products (resulting from the transformation of atomic nuclei emitting radiation)
Source: Japan Radioisotope Association, "Radioisotope Pocket Data Book 11th Edition"